Platinum Prints
Joan M. Walker and Constance McCabe

Alfred Stieglitz, John Marin, 1911, platinum print, National Gallery of Art, Washington, Alfred Stieglitz Collection, 1949.3.419
Key Set number 369
View all platinum prints in the Key Set
Alfred Stieglitz’s process of choice from the late 1880s to the early 1920s was the platinum print, or “platinotype.” An early adopter of the platinotype, he celebrated the process both for its aesthetic qualities and reputation for permanence.
The platinum print process is based on the characteristics of light-sensitive iron salts, which react with platinum salts to form platinum metal. A sheet of paper is coated with a solution of these salts to make it sensitive to light. Once dry, the sensitized paper is exposed to light through a negative, developed in a chemical solution, cleared, and washed. The print’s hue may range from charcoal gray to sepia depending on the chemical properties of the sensitizer and developer. Various image hues can also be achieved by adjusting the moisture content of the sensitized paper and/or the temperature at which a print is developed. Further chemical additions to the sensitizer and/or developer provide seemingly endless options for fine-tuning the appearance of the photograph.
The first commercially manufactured platinum paper was introduced in 1879 by British inventor William Willis Jr., who perfected the process over the following decades, gradually expanding the variety of his Platinotype Company products. A chemical variant of the platinum process was introduced in 1887 by Austrian Giuseppe Pizzighelli and marketed by several manufacturers in Europe and the United States.
Unlike a gelatin silver print, the image in a platinum print is absorbed directly into the paper, taking on its characteristics. Thus, the paper upon which a platinum print is made affects the quality of the image. An untreated paper will yield a matte print with a softer and less-detailed image than one made, for example, on a paper that has been chemically modified to impart a sheen, such as the Platinotype Company’s Japine papers, first introduced in 1906.
Stieglitz used both matte and Japine papers until manufacturers decreased production of platinum papers during World War I due to restrictions on the use of platinum for nonmilitary purposes. In 1918, he voiced the frustrations experienced by devotees of the platinum print: “A kingdom for some decent platinum paper. Just a few sheets. I see I have run out of paper” (Alfred Stieglitz to Paul Strand, August 22, 1918, Alfred Stieglitz/Georgia O’Keeffe Archive, Beinecke Rare Book and Manuscript Library, Yale University).
A Closer Look: Two Platinum Print Processes
Like others of the time, Stieglitz preferred platinum papers’ matte surface and range of image tones, and he experimented extensively with both Willis and Pizzighelli commercial platinum papers. From 1887 to 1902, he wrote eighteen articles on the platinum processes in which he not only shared numerous recipes for hand-sensitizing papers, but also reviewed commercial products and explained how chemical manipulations could alter the tone of the image from neutral gray-black to sepia.
Willis’s process requires chemical development to complete the formation of a black image; raising the temperature of the developer could produce a warm black image, but a mercury additive was required to achieve a true sepia tone. Pizzighelli’s process, also known as the “direct platinum” or “water-developed” process, requires no chemical development. Only the presence of water, as steam or moisture in the sensitized paper, is required to form the image. In his 1891 essay “Tints of Prints Made on Direct Printing Platinotype Paper,” Stieglitz noted that the tone of the final print could be controlled by adjusting the moisture content of the sensitized paper prior to exposure. If the paper was moistened prior to exposure, a black image would result; if the paper was dried prior to exposure, a sepia image would result.
Three of Stieglitz’s early platinum prints that were made from the same negative (Sunlight Effect, Gutach) illustrate how he exploited the chemical properties of the process to achieve different image appearances. These examples make particularly useful comparisons and provide insight into the young Stieglitz’s artistic choices and practices.

Alfred Stieglitz, Sunlight Effect, Gutach, 1894, printed 1895–1896, platinum print mounted on laid paper, National Gallery of Art, Washington, Alfred Stieglitz Collection, 1949.3.174
Key Set number 186

Alfred Stieglitz, Sunlight Effect (Gutach), 1894, printed 1895/1896, platinum print, National Gallery of Art, Washington, Patrons’ Permanent Fund, 2006.3.1.13

Alfred Stieglitz, Sunlight, 1894, printed 1895/1896, platinum print, National Gallery of Art, Washington, Alfred Stieglitz Collection, 1949.3.175
Key Set number 185
It is possible to detect the metal components of a photograph using a non-sampling, non-destructive analytical method known as x-ray fluorescence spectrometry (XRF). In the case of the three examples of Sunlight Effect, Gutach, platinum is detected in all of the prints. However, the very warm-toned sepia print (Key Set number 185) also contains mercury. Mercury salts were commonly used as additives to both the sensitizer and developer to achieve sepia platinum prints. The lack of mercury in the other warm-toned version indicates that it was printed using Pizzighelli’s direct platinum process and the warm hue was achieved by drying the sensitized paper prior to exposure.
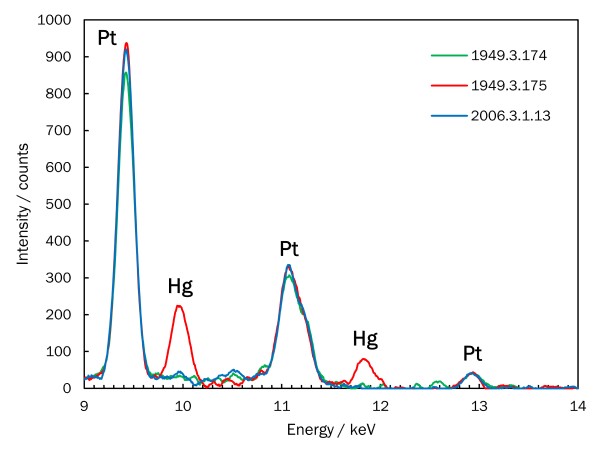
Detail of XRF spectra, three prints of Sunlight Effect, Gutach
This detail of the XRF spectra for the three prints of Sunlight Effect, Gutach shows that all three contain platinum (Pt) as the primary image metal. Only the sepia-hued print (1949.3.175, Key Set number 185) also has mercury (Hg) in the print, which gives it its warm hue.
Suggested Reading
McCabe, Constance, ed. Platinum and Palladium Photographs: Technical History, Connoisseurship, and Preservation. Washington, DC: American Institute for Conservation of Historic and Artistic Works, 2017.