Carbon Prints
Courtney Helion, Joan M. Walker, and Constance McCabe

Alfred Stieglitz, The Net Mender, 1894, carbon print, National Gallery of Art, Washington, Alfred Stieglitz Collection, 1949.3.199
Key Set number 212
View all carbon prints in the Key Set
Unlike silver and platinum printing methods that rely on the light-sensitive properties of metal salts to form a metallic image, the carbon process depends on the light sensitivity of dichromated gelatin. This material hardens in proportion to the amount of light it receives, forming an image that consists of pigment in gelatin. To make a typical carbon print, a sheet of paper is coated with a solution of gelatin, potassium dichromate, and pigment. Once dry, this light-sensitive “carbon tissue” is placed in contact with a negative and exposed to light, locally hardening the carbon tissue. The exposed tissue is then transferred to a paper support by wetting both papers, placing the tissue face down onto the new paper, and squeegeeing the pigmented film into firm contact. Under water, the exposed carbon tissue is carefully peeled away. The unexposed pigmented gelatin dissolves in the bath, and the positive carbon print is revealed on the new paper support.
Introduced in 1855 by French chemist Alphonse-Louis Poitevin, and perfected in 1864 by British chemist and physicist Joseph Swan, the carbon process was moderately popular throughout the latter part of the nineteenth century. The Autotype Company of London was the predominant firm that made and sold these tissues commercially. They were available in a variety of hues, in addition to the “carbon” black from which the name of the process derives, capable of fine detail and a lustrous surface.
Alfred Stieglitz was a champion of the carbon printing process, claiming, “On artistic grounds, there is but one printing process which holds its own with the platinotype, and that is carbon” (American Amateur Photographer, 1892). He believed it to be “undoubtedly the most beautiful of the photographic processes, although not as simple as the platinotype” (American Amateur Photographer, 1893). He made and widely exhibited carbon prints in a variety of hues during the mid- to late 1890s.
The eleven carbon prints in the Key Set have characteristics that are both similar to and different than other processes used by Stieglitz. Like his early platinum prints, his carbon prints range in hue from warm browns to neutral blacks and grays. But unlike his platinum prints, which are on smooth or slightly textured paper, he chose heavyweight, rough-textured watercolor papers to make his carbon prints. Stieglitz’s print A Wet Day on the Boulevard, Paris shows the surface texture of the watercolor paper.

Alfred Stieglitz, A Wet Day on the Boulevard, Paris, 1894, carbon print, National Gallery of Art, Washington, Alfred Stieglitz Collection, 1949.3.108
Key Set number 114

Detail of A Wet Day on the Boulevard, Paris, in normal light (left) and specular light (right). The detail in specular light illustrates the rough texture of the paper.
A Closer Look: Identifying Carbon Prints
The darker regions of most carbon prints appear glossier than the lighter tones. These dark areas are thicker than the lighter regions because they received more light, and thus more gelatin was hardened, during exposure. This image relief, while sometimes subtle, is a characteristic that helps to identify a carbon print.
Stieglitz’s An Icy Night provides visual evidence of the transfer of the carbon tissue to produce the final print: a bubble was trapped between the tissue and the receiving paper, burst when it was squeegeed flat, and was subsequently retouched to diminish the appearance of the flaw.

Alfred Stieglitz, An Icy Night, 1898, carbon print, National Gallery of Art, Washington, Alfred Stieglitz Collection, 1949.3.254
Key Set number 257

Detail of An Icy Night showing burst and retouched bubble
Technical analysis can also assist in identifying carbon prints. Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) analysis performed on Winter, Fifth Avenue detected gelatin at the surface of the print. Elemental analysis by x-ray fluorescence spectrometry (XRF) found that more chromium is present in the darker areas. Taken together, these results provide strong evidence that the image was formed with dichromated gelatin, indicating that the carbon print process was used.

Alfred Stieglitz, Winter, Fifth Avenue, 1893, printed 1894, carbon print, National Gallery of Art, Washington, Alfred Stieglitz Collection, 1949.3.93
Key Set number 82
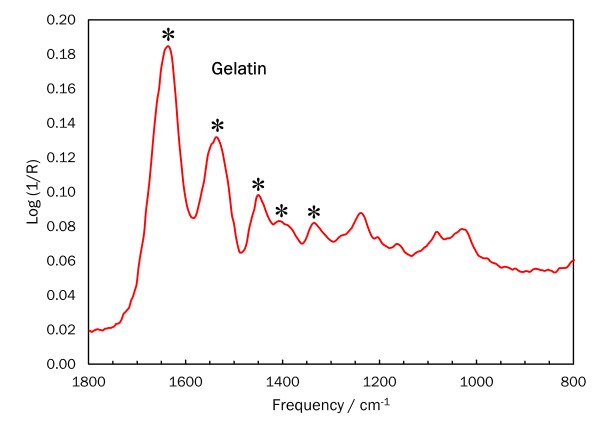
ATR-FTIR spectrum for Winter, Fifth Avenue
The ATR-FTIR spectrum shows peaks characteristic of gelatin, indicated with an asterisk (*) in the figure.
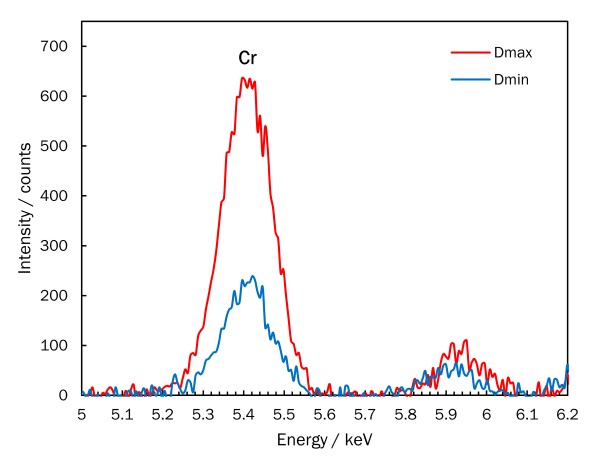
XRF spectra for Winter, Fifth Avenue
The signal for chromium (Cr) detected by XRF is more intense in the darker image areas (Dmax) than in lighter image areas (Dmin).